Scientists have been working for decades to create synthetic cells that mimic key functions of biological cells. The challenge has been to make the artificial cells dynamic, responsive, and capable of interacting with their environment in a controlled way. A new study published in Nature Materials, ‘Morphology remodelling and membrane channel formation in synthetic cells via reconfigurable DNA nanorafts’ (authored by Sisi Fan, Shuo Wang, Longjiang Ding, and others), presents a breakthrough involving the use of DNA nanorafts — tiny, flat assemblies of DNA strands that change shape in response to chemical signals.
By using DNA as a programmable building material, researchers have found a way to engineer membranes that can change shape, open and close transport pathways, and respond to specific molecular signals.
Molecular marvel
DNA’s ability to form predictable and programmable interactions makes it an ideal material for building nanoscale devices, such as the nanorafts used in this study.
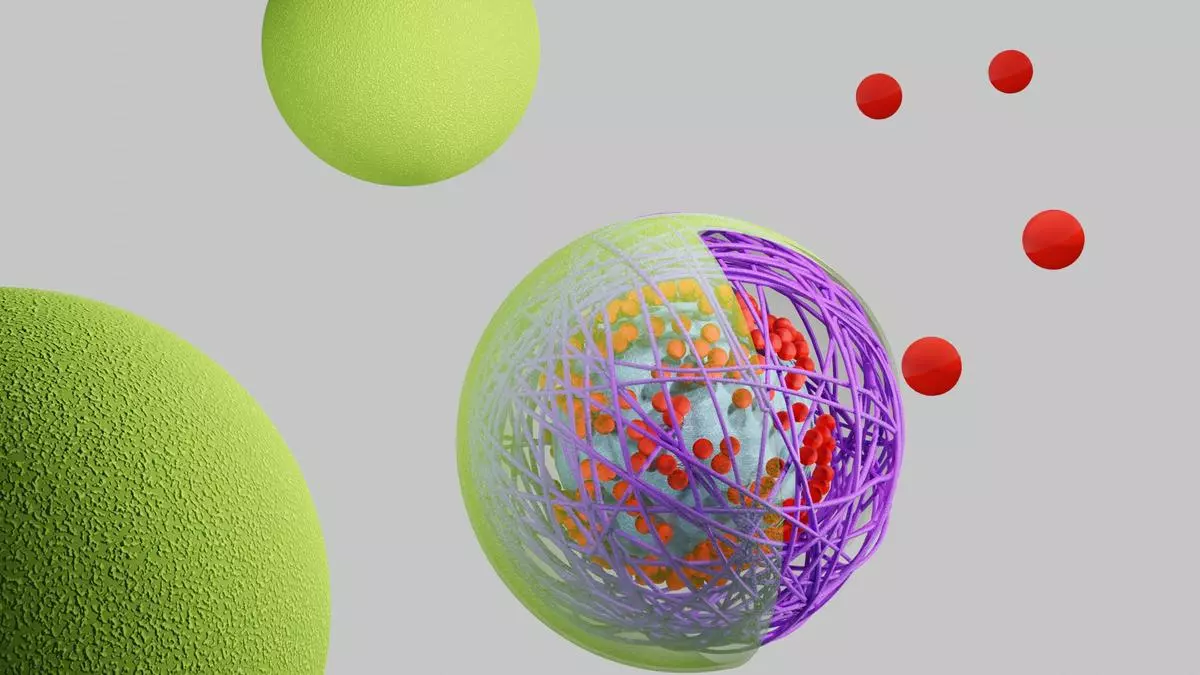
The nanorafts were integrated with giant unilamellar vesicles (GUVs), which serve as simplified models of biological cell membranes. By attaching DNA nanorafts to the surface of these synthetic cells, scientists were able to induce and control changes in their membrane structure. Membrane shape is critical to many cellular processes, including transport, engulfing nutrients, sending signals, moving through tissues, and division.
The innovation here is that the DNA nanorafts are not passive structures. They are designed to switch between different shapes and, in doing so, they actively influence the behaviour of the membranes they are attached to, marking a major advance in synthetic biology. It opens up new possibilities for engineering artificial life-like systems.
Cell shaping
Initially the nanorafts are in a square-like conformation; but when exposed to specific DNA strands called ‘unlocking strands’, they elongate into a rectangule, which applies force on the membrane and deforms it in a controlled way.
Equally important is the ability to reverse these shape changes using a different set of ‘locking strands’.
Gatekeeping
Beyond reshaping membranes, the DNA nanorafts enable another crucial function: forming and sealing synthetic channels within the membrane. Transporting molecules across cell membranes is one of the fundamental functions of life, allowing cells to absorb nutrients, remove waste, and communicate with their surroundings. In biological cells, this process is carried out by protein-based channels and transporters. However, engineering such complex protein structures has always been a challenge in synthetic biology.
The study offers DNA-based structures as an alternative. When the nanorafts elongate, they interact with the membrane in a way that creates openings or pores. These synthetic channels allow in molecules as large as 70 kilodaltons (kDa), which is not possible in most natural protein channels.
Importantly, these pores are not permanent. Once the membrane regains its original shape, the pores close and seal off the transport pathway. This control over membrane permeability is a major step forward in creating artificial cells that can selectively regulate molecular transport.
Applications
The ability to reshape membranes and create selective transport channels has significant implications for drug delivery, bio-sensing, and artificial cell research. Imagine a synthetic cell that can absorb a drug molecule in one environment, travel through the body, and release it at a specific site, such as a tumour. Programmed DNA nanorafts promise highly precise drug delivery with minimal side effects.
Synthetic cells can also serve as biosensors to detect disease markers, toxins, or bacterial infections by responding to specific molecules and changing their membrane structure accordingly. They could provide real-time medical and environmental diagnostics.
In fundamental research, DNA nanorafts can help us understand how biological membranes function and gain new insights into the fundamental principles of cell biology.
While natural cells have evolved complex mechanisms for regulating their shape and transport processes, the new findings suggest that we can design artificial systems with similar and, potentially, enhanced capabilities.